Quality Control Log: Ensuring Laboratory Excellence in 2024
A quality control log is a tool used to track, analyze, and improve lab processes, ensuring consistent and accurate test results.
Brannon Hogue
7/29/20242 min read


I. Introduction
Quality control logs are important for maintaining consistent test results in the lab. Using it as a tool to track, analyze, and improve your lab processes is the most important way to utilize the quality control logs in your lab. Identifying issues early is always the best way to ensure everything runs smoothly and the machines are working properly.
II. Key Components of a Quality Control Log
A. Date and time
B. Product or process information
C. Inspector details
D. Parameters checked
E. Specifications and actual values
F. Pass/fail status
G. Corrective actions
H. Notes or comments
We have a free template you can download and change based on your labs specifications.
III. Implementing an Effective Quality Control Log System
To maximize the implementation of these QC procedures, you should do the following:
I. Establish crystal clear SOP’s in policy book. If you use medialab that is even better because referencing it will be easier to do than if you use paper binders of your policy book.
II. Train technicians so that they are not guessing. The QC logs do not work well if you do not know how to use them
III. Make QC logging built in to the daily workflow
VI. Implement schedules, they could be electronic or on sticky notes for technicians to see.
IV. Benefits of Using Quality Control Logs
Most labs are already required to run QC tests based on manufacturing specification. There are several benefits to going above and beyond with quality control logs, including improving accuracy, ensuring extra compliance with regulatory standards, and identifying trends that can lead to process improvements. Additionally, well-maintained QC logs can serve as valuable documentation during audits and inspections. They also help in maintaining a high level of customer satisfaction by consistently delivering quality products.
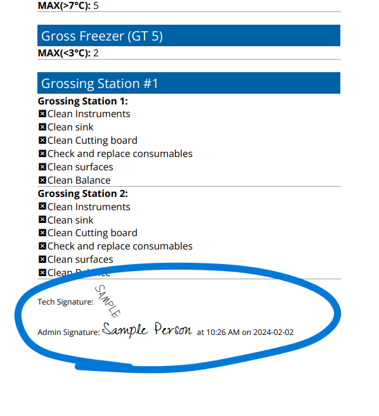

Build custom QC forms to match your lab’s unique workflow requirements
Record any data type on electronic tablet in place of paper forms
Entered data is compared to reference ranges in real-time.
Out-of-range QC data automatically triggers mandatory corrective action events
All forms require full completion and signature before submission(Show in the picture)
Supervisors can review and sign submitted forms from their desk or device
Digitize your labs daily and weekly equipment QC checklist
V. Digital vs. Paper-Based Logs
Most modern lab equipment performs QC automatically, but LabQCPro can digitize your maintenance QC logs, streamlining the process and ensuring comprehensive documentation.
VI. Conclusion
In summary, quality control logs are essential tools for maintaining and improving lab processes. By implementing effective QC log systems, labs can ensure accurate results, regulatory compliance, and continuous process improvement.


Contact Us
915 Deedra Avenue Pensacola, FL 32502 United States
(850) 696-7438
info@labqcpro.com
